MALARIA LIFE CYCLE
Table of Contents
Here we discuss the malaria life cycle.
Malaria is caused by five kinds of single-cell eukaryotic Plasmodium parasites (mostly Plasmodium falciparum or Plasmodium vivax) that are transferred to humans by the bite of Anopheles.
Malaria parasites develop and multiply in human liver cells initially, then in red blood cells exponentially. Malaria symptoms in humans are caused by the cell cycle of the parasite lifecycle. Malaria is commonly divided into three categories: asymptomatic, uncomplicated, and severe. All Plasmodium species can induce asymptomatic malaria, which means the patient possesses circulating parasites but still no symptoms. All Plasmodium species can produce uncomplicated malaria.
The symptoms usually appear 7-ten days after the insect bite. Fever, mild to severe shaking chills, profuse perspiration, headache, nausea, vomiting, diarrhea, and anemia is some of the non-specific symptoms, with no clinical or laboratory indications of serious organ dysfunction.
Plasmodium falciparum infection is the most common cause of severe malaria, but it can also be caused by Plasmodium vivax and Plasmodium know. Severe anemia and end-organ damage, such as coma (cerebral malaria), respiratory problems (such as edema and hyperpneic syndrome), and hypoglycemia or acute renal injury, are all possible complications. Malaria is frequently accompanied by hyperparasitaemia, which is linked to an increased risk of death. On the Severe Malaria Observatory, you may learn more about the epidemiological criteria of severe malaria.
Malaria can be Found Almost Anywhere
Malaria is most commonly found in tropical and sub-tropical regions in warmer parts of the world. The Anopheles mosquito thrives at warmer temperatures. Malaria parasites, which continue to develop inside the insect, require the temperature to finish their development before they can be transmitted to people.
Malaria can be found in over 100 nations and territories. Approximately half of the world’s population is endangered. Malaria transmission occurs across large parts of Africa and South Asia, as well as parts of South America, the Mediterranean, Southeast Asia, the Mideast, and Oceania. Malaria, on the other hand, does not occur in all warm climates. Malaria has been eradicated in some nations with warm climates, whereas malaria is absent in a few others due to the absence of Anopheles mosquitoes.
What is the Difference Between the three Phases of malaria?
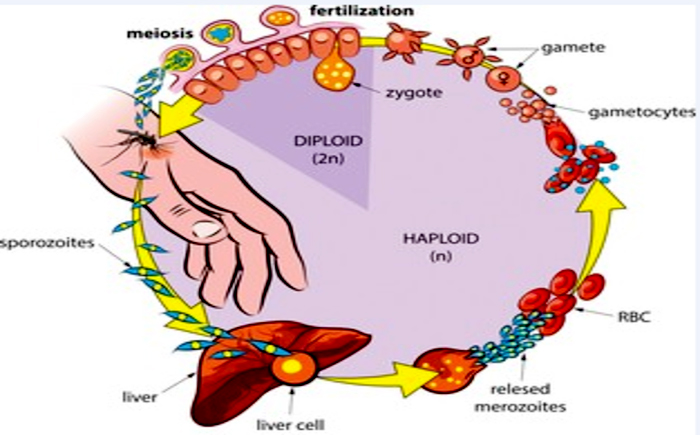
Whenever the parasite infects an animal, it attacks in three parts: initially, it affects liver cells, then blood cells, and ultimately, it generates gametes that mosquitos can transmit. The majority of therapies focus on parasites in the cell cycle, which causes the symptoms of malaria fever, vomiting, and coma. Stuart L. is a writer who lives in the United States.
Humans and female Aphid mosquitoes are infected cyclically in the evolutionary biology of malaria. The parasites in people grow and reproduce first in liver cells, and in the red blood cells. In the bloodstream, successive broods of parasites invade and destroy red cells, releasing daughter parasites (merozoites) that continue the cycle by infecting additional red cells.
Malaria symptoms are caused by parasites that dwell in the bloodstream. When some types of blood-stage worms (gametocytes, which come in both male and female forms) are ingested by an Anopheles mosquito during blood feeding, they pair up in the mosquito’s gut and begin a growth cycle and reproduction. After 10-18 days, the parasite’s sporozoite form migrates to the insect’s salivary glands.
When an Anopheles mosquito feeds on another person’s blood, anticoagulant saliva is injected along with sporozoites, which travel to the liver and start a new cycle. As a result, an infected mosquito spreads the disease out of one human to the next (serving as a “vector”), while sick humans pass the parasite on to the mosquito. In contrast to the host body, the mosquito vector is unaffected by the parasites’ existence.
Mosquitoes of the Anopheles Genus
The malaria life cycle of Anopheles mosquitoes is divided into four stages: egg, larva, pupa, and adult. Mosquitoes lay their eggs in a range of fresh and brackish water sources. As a result, the first three phases are aquatic in nature and last between 7 and 14 days. Malaria is usually spread by the older anopheles mosquitoes. The species Anopheles existing in a given location at a given time, on the other hand, has an impact on the severity of malaria incidence.
The parasite Plasmodium falciparum, which seems to be a parasite, can produce a more severe type of malaria that can even lead to death. The malaria life cycle is divided into these parts.
- Malaria infection starts when a female mosquito bites a human, injecting malaria parasites into the bloodstream in the shape of sporozoites.
- The sporozoites enter the human liver soon.
- Over the next 7 to 10 days, the sporozoites grow asexually inside the liver cells, generating no symptoms.
- In an animal study, the parasites are expelled from the liver in vesicles, travel through the heart, then arrive inside the lungs, when they settle within pulmonary capillaries as merozoites. The vesicles gradually disintegrate, allowing the merozoites to go to the blood stage of development.
- Merozoites infect blood cells (erythrocytes) in the bloodstream and grow until the cells explode. After that, they infiltrate additional erythrocytes. Each time worms break loose and invade blood cells, the cycle repeats, generating fever.
- Some infected blood cells break the asexual multiplication cycle. Instead of reproducing, the oocysts in these cells mature into gametocytes, which circulate in the bloodstream as sexual versions of the parasite.
- Whenever a mosquitoes bites a human, the gametocytes are ingested, and the gametocytes develop into adult sex cells known as gametes.
- Fertilized female gametes evolve into urgent ookinetes which burrow through the midgut wall of the mosquito and create oocysts on the outer surface.
- Millions of active sporozoites from inside the oocyst. When the oocyst explodes, sporozoites are released into the bodily cavity and proceed to the insect’s salivary glands.
- When a mosquitoes bites another person, the human infection cycle starts all over again.
The merozoite’s specialized apical secretory organelles, known as micronemes, rhoptries, or dense granules, are responsible for the merozoite’s attachment, invasion, or establishment inside the red cell. The parasite’s initial engagement with the red cell causes a quick “wave” of distortion across the red blood cell membrane, resulting in the development of a stable parasitic organism cell connection.
The parasite then uses the actin-myosin motor, proteins from the thrombospondin-related anonymous family proteins (TRAP), and aldolase to push its way through the erythrocyte bilayer and create a parasitophorous phagocyte to seal itself off from the host-cell cytoplasm, creating a favorable environment for its advancement inside the red cell. The parasite shows up as an intracellular “ring” at this stage.
Formalized paraphrase with the continuous cycling of a parasite population, parasite numbers increase fast within red cells. The lack of conventional biosynthetic routes and intracellular organelles in red cells tends to cause barriers for fast-growing intracellular parasites, despite the fact that red cells provide a little immunological benefit to the growing parasite. The growing circle stages overcome these impediments through a variety of mechanisms, including nutrient restriction to the abundant hemoglobin, the dramatic growth of the surface area via the formation of a tubovesicular system, and the export of a variety of remodeling and infectivity factors into the red cell.
Formalized paraphrase Hemoglobin from the erythrocytes, the parasite’s main source of nutrition, is consumed and digested in a feeding vacuole. The remaining hazardous heme is detoxified via heme polymerase and sequestered as hemozoin, and the amino acids so made available are used for protein production (malaria pigment). The parasite gets its energy from anaerobic glycolysis, which involves enzymes like pLDH and plasmodium aldolase.
The transmembrane permeability and cytosolic makeup of the host cell change when the parasite grows & multiplies within the red cell. Formalized paraphrase these novel permeation channels created in the cell membrane by the parasite aid not just in the uptake of external solutes, but also in the elimination of metabolic waste products and the formation and maintenance of electrochemical ion gradients.
At the same time, the inordinate ingestion, digestion, as well as detoxification of a host cell’s hemoglobin, as well as its discharge out of the diseased RBCs through the new permeability pathways, prevents premature hemolysis of a highly passivated layer of infected red cells, preserving the infected red cells’ osmotic stability. In the case of P. knowlesi, the erythrocytic cycle lasts 24 hours, 48 hours in the case of P. falciparum, P. vivax, and P. ovale, and 72 hours in the case of P. malariae.
Each clear definition grows and divides into 8–32 (average 10) new merozoites within the vacuole during each cycle, passing through the phases of ring, trophozoite, then schizont. The infected RBCs break at the conclusion of the cycle, releasing new merozoites, which then infect other RBCs. The parasite populations can quickly rise to as high as 1013 each host as a result of unbridled growth. Formalized paraphrase. A tiny percentage of asexual worms do not go through schizogony and instead develop into gametocytes in the sexual stage.
These extracellular, nonpathogenic man or woman gametocytes aid in the transmission of infection to others by the female anopheline mosquito, where they continue the parasite’s sexual phase of the life cycle. P. vivax gametocytes develop shortly after merozoites are released from the liver, whereas P. falciparum gametocytes develop much later, with peak sexual stage densities often occurring one week after peak androgynous stage densities.
What are malaria’s long-term consequences?
Anemia (poor blood) may develop, as well as hepatitis (liver failure) and the passage of hemoglobin (blood) in the urine. Some patients may experience more serious consequences, such as aberrant body posture, irregular eye motions, ocular movement paralysis, and coma.
Who is in danger?
Malaria posed a threat to over half of the world’s people in 2019. In Sub-Saharan Africa, the majority of malaria cases and mortality occur. South-East Asia, the Eastern Mediterranean, the Western Pacific, as well as the Americas are all at risk, according to the WHO.
Some people are at a far higher risk of catching malaria and having a serious illness than others. Infants, children under the age of five, pregnant women, HIV/AIDS patients, non-immune migrants, mobile groups, and travelers are among them. Malaria control programs must take extra precautions to safeguard these vulnerable populations from falciparum malaria, taking into account their unique circumstances.
Prevention is the Best
The primary method for preventing and reducing malaria transmission is vector control. A measure of protection will be provided across the community if the coverage of pest control actions within a certain area is high enough.
All people at risk of malaria should be protected with effective vectors of malaria control, according to the WHO. Pesticide mosquito nets and residual spraying are two types of vector control that work in a variety of situations.
Malaria Treatment and Evaluation
Malaria can be reduced and deaths can be avoided if diagnosed and treated early. It also aids in the reduction of malaria transmission. The current best treatment, especially for P, is an artemisinin-based combination therapy.
Before starting malaria treatment, the WHO recommends that all cases of probable malaria be confirmed by parasite-based medical tests (either microscopy or quick diagnostic tests). In 30 minutes or less, parasitological confirmation results can be obtained. When a serologic diagnosis is not attainable, treatment based purely on symptoms should be considered. The revised WHO Recommendations for Malaria contain more detailed advice.
And what’s the new malaria treatment?
Krintafel (tafenoquine), a novel malaria medicine, inhibits relapse caused by the protozoan vivax (P. vivax), along with several other parasites that cause the disease. Patients with P. vivax actually require a 10-day treatment regimen, which many do not finish, resulting in malaria recurrence.
Why isn’t quinine used to treat infections anymore?
Health-related.Since there are other medicines that are similarly efficient and have fewer side effects, the World Health Assembly (WHO) no longer recommends quinine as a first-line drug for the treatment as of 2006. It should only be used when artemisinins are unavailable, according to the researchers.
Which antibiotics are used for malaria treatment?
Travelers to any malaria-endemic areas can take doxycycline for malaria treatment prevention. Doxycycline can be used to treat infections when combined with other drugs.
Response from the World Health Organization
Malaria a global technical strategy from the World Health Organization (WHO) for 2016-2030
The World Health Organization endorsed the WHO Global technical plan for malaria 2016-2030 in May 2015, which provides a technical foundation for all disease countries. Its purpose is to advise and coordinate country-level malaria control and elimination efforts.
The Strategy establishes a number of global goals that are both ambitious and attainable, including:
- Decreasing the number of malaria cases by at least 90% until 2030
- By 2030, malaria fatality rates will have fallen by at least 90%
- By 2030, malaria will have been eradicated in at least 35 nations
- Avoiding the comeback of plasmodium in all malaria-free countries
This Strategy was the outcome of a two-year consultative process that included more than 400 technical specialists from 70 Member States. In May 2021, the World Health Assembly approved a revised version of the Strategy through resolution WHA74.9, which was released in 2021. It reflects the worldwide malaria response’s learnings in the intervening years. While the objectives and goals remain the very same, the tactics for combating malaria have evolved in some regions to keep up with the changing terrain.
Malaria No More is a global malaria prevention and control initiative
The WHO Global Malaria Programme oversees WHO’s global attempts to control or eliminate malaria by: • developing, disseminating, and promoting evidence-based norms, standards, strategies, technical methods, and guidelines; and
- Keeping a separate tally of global progress
- Establishing capacity-building, system-strengthening, and surveillance strategies
- Finding new areas for change as well as risks to malaria management and elimination
The Malaria Policies Advisory Panel (MPAC), a team of global malaria specialists established after an open nomination procedure, supports and advises the Programme. MPAC’s mandate is to give strategic advice and technical input on all elements of malaria elimination in the context of an open, responsive, and credible policy-making process.
“A high-burden, high-impact strategy”
Dr. Tedros Adhanom, MD The WHO Director-General urged an aggressive novel strategy to jump-start success against malaria at the World Health Organization in May 2018. In November 2018, Mozambique introduced a new country-led approach called “High Burden, High Impact.”
The 11 countries with the highest illness burden are now driving the strategy
The following are important factors to consider:
- There is a political will to prevent malaria deaths
- Data strategy for maximum impact
- Improved direction, policies, and strategies
- A national malaria response that is well-coordinated
“High burden to high impact,” a project led by the RBM Partnership for End Malaria, is based on the idea that no one should perish from a disease that can be prevented and detected, and that is completely curable with existing malaria treatments.
FAQ
How long would it take to be malaria-free?
Malaria symptoms normally fade away fast with good treatment, and the disease is usually cured within two weeks. Malaria bouts (fever, chills, sweating) can recur over a period of years if not treated properly. Patients would become partially resistant and get a lesser illness with repeated exposure.
Is plasmodium a virus or a parasite?
Malaria is caused by a parasite, not a disease or bacteria. Malaria is a disease caused by the Plasmodium parasite, which is generally spread by infected mosquitoes. A mosquito feeds on the blood of an infected person, ingesting Plasmodia that are present in the blood.
When you have malaria, how do you feel?
Malaria makes people feel very sick, with a rising fever and shivering chills. Malaria is still frequent in tropical and subtropical nations, despite its rarity in temperate settings. Malaria infects over 290 million people each year, with over 400,000 people dying as a result of the disease.
How does the body defend itself against malaria?
When parasites mature in blood cells, the immune system finds and recognizes them. Immune cells are removed to brutally murder parasites in order to protect the body. I hope you understand all about the malaria life cycle.
A respected health writing specialist recognized all over the globe, together with Aneeza, created by medshelper.com